Autophagy is a natural process where cells break down and recycle their own components. The word comes from Greek, meaning “self-eating.” Though it may sound harmful, this process is essential for keeping cells healthy. It helps clear out damaged parts and reuses them for energy and repair.
This process becomes especially important when the body is under stress, such as during infection, starvation, or other challenges. Autophagy helps cells survive tough conditions by removing harmful material and maintaining balance inside the cell. Without it, waste would build up and interfere with normal cell function.
In recent years, scientists have learned that autophagy does more than basic cleanup. It also plays a role in development, the immune system, aging, and many diseases. Problems with autophagy have been linked to cancer, brain disorders, and metabolic conditions. Understanding how it works is helping researchers find new ways to treat these health issues.
The Basics of Autophagy
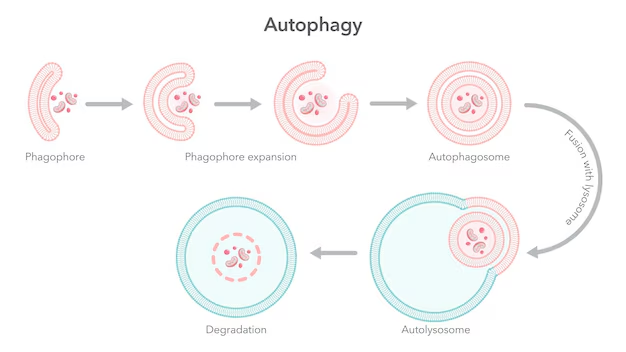
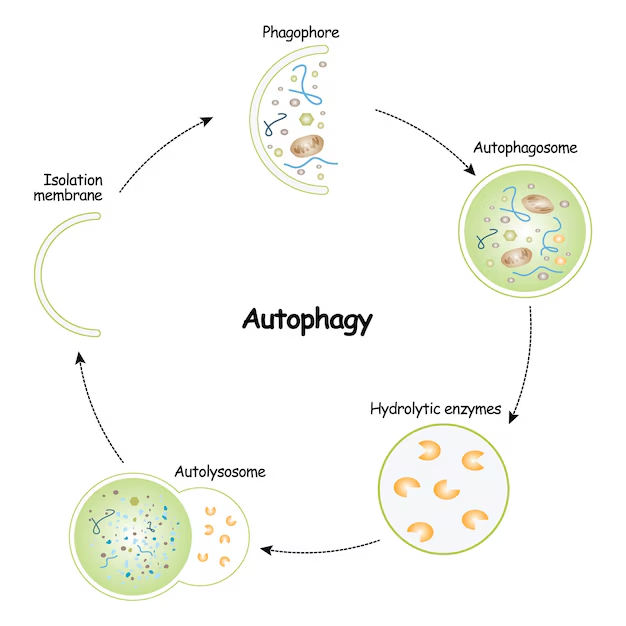
Autophagy is a catabolic process where cytoplasmic components, such as damaged organelles, protein aggregates, and invading pathogens, are sequestered into double-membrane vesicles called autophagosomes. These autophagosomes then fuse with lysosomes, forming autolysosomes, where the contents are degraded by lysosomal enzymes and the resulting macromolecules are recycled.
Types of Autophagy
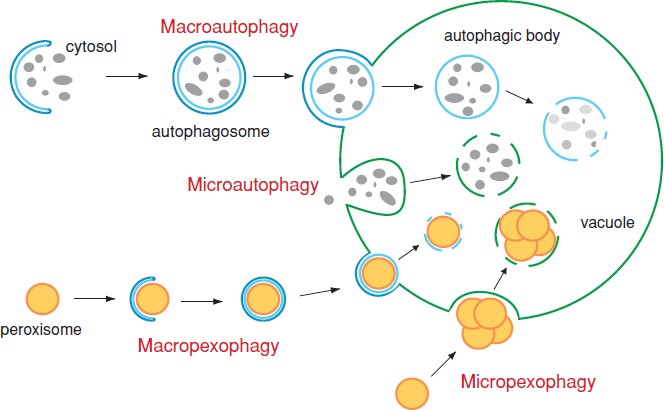
There are three main types of autophagy:
Macroautophagy: The most studied form, where cytoplasmic cargo is enclosed by autophagosomes.
Microautophagy: Direct engulfment of cytoplasmic material by the lysosome through membrane invagination.
Chaperone-mediated autophagy (CMA): A selective process where specific proteins are recognized by chaperones and translocated across the lysosomal membrane.
Among these, macroautophagy (commonly referred to as just “autophagy”) is the most comprehensively studied and is often implied when the term is used without qualification.
Molecular Mechanism of Autophagy
Autophagy happens through a carefully controlled series of steps, each guided by specific proteins. These steps ensure that damaged or unwanted cell parts are safely enclosed, broken down, and recycled. This process keeps cells clean and working properly, especially during times of stress or damage.
Many of the proteins involved in autophagy were first discovered in studies using yeast. Researchers later found that these same proteins also exist and function similarly in mammals, showing how this process is preserved across different species. This discovery has helped scientists understand how autophagy works in human health and disease, opening the door to new research and possible treatments.
Key Stages
Initiation: Triggered by cellular stress or nutrient deprivation, the ULK1 complex (comprising ULK1/2, Atg13, FIP200) is activated.
Nucleation: The class III PI3K complex (Beclin-1, Vps34, Vps15, Atg14L) initiates the formation of the phagophore, the precursor to the autophagosome.
Elongation and Closure: Two ubiquitin-like conjugation systems facilitate the expansion of the phagophore:
Atg12–Atg5–Atg16L1 complex
LC3 (microtubule-associated protein 1A/1B-light chain 3), which is lipidated to form LC3-II and incorporated into the autophagosomal membrane.
Fusion with Lysosome: Mature autophagosomes fuse with lysosomes to form autolysosomes.
Degradation and Recycling: Lysosomal enzymes degrade the contents, releasing amino acids, fatty acids, and sugars back into the cytoplasm.
Regulation of Autophagy
Autophagy is closely controlled by the cell’s environment, especially the levels of nutrients, energy, and stress. When nutrients are low or the cell is under stress, autophagy becomes more active to help the cell survive by recycling old or damaged parts. This balance is important for maintaining overall cell health.
Two important regulators that control this process are mTOR and AMPK. mTOR acts as a sensor for nutrients and energy; when nutrients are plenty, it suppresses autophagy. On the other hand, AMPK is activated during low energy conditions and helps promote autophagy. Together, they help the cell decide when to start or stop the recycling process, depending on what the cell needs to stay healthy.
mTOR (mechanistic target of rapamycin): A nutrient-sensing kinase that inhibits autophagy under nutrient-rich conditions.
AMPK (AMP-activated protein kinase): Activated during low energy states; promotes autophagy by inhibiting mTOR and activating ULK1.
Hormones like insulin, glucagon, and stress-related signals (e.g., oxidative stress, hypoxia) also modulate autophagy through complex signaling networks.
Physiological Roles of Autophagy
A. Cellular Homeostasis
Autophagy helps maintain a clean and balanced internal environment within the cell by removing damaged proteins, broken organelles, and other waste materials. This process is especially important in non-dividing cells like neurons, where cellular waste can build up over time and cause problems. By keeping the cell clear of harmful debris, autophagy supports long-term cellular function and health.
B. Development and Differentiation
Autophagy plays an important role during early stages of life, including embryonic development and the formation of specialized cells. It helps shape tissues and organs by clearing away unneeded or defective parts of cells. This ensures that cells grow and change properly during development and that tissues form in the correct way.
C. Immunity and Inflammation
Autophagy supports the immune system by removing harmful microbes from inside cells in a process known as xenophagy. It also helps regulate the body’s inflammatory response by breaking down inflammasomes—protein complexes that trigger inflammation—and controlling the production of cytokines, which are signaling proteins that affect immune activity.
D. Metabolism and Energy Balance
When the body is low on food or energy, such as during fasting, autophagy steps in to recycle internal resources. It breaks down parts of the cell to release nutrients that can be used for energy. This supports key processes like gluconeogenesis (making glucose) and lipid metabolism (breaking down fats), helping the body stay balanced during times of nutrient shortage.
Autophagy in Health and Disease
While basal autophagy is essential for health, dysregulated autophagy is associated with many diseases. It can be either protective or detrimental depending on the context.
A. Neurodegenerative Diseases
Conditions like Alzheimer’s, Parkinson’s, and Huntington’s disease involve the accumulation of misfolded proteins and defective organelles, often due to impaired autophagy. Enhancing autophagy is a promising therapeutic approach to clear toxic aggregates and improve neuronal survival.
B. Cancer
Tumor suppression: In early stages, autophagy prevents genomic instability by removing damaged organelles and proteins.
Tumor promotion: In established cancers, autophagy can support tumor cell survival under stress and contribute to resistance to chemotherapy.
Some therapies aim to inhibit autophagy in tumors, while others attempt to boost it to eliminate precancerous cells.
C. Cardiovascular Diseases
Autophagy maintains cardiac cell health by removing damaged mitochondria (mitophagy). Dysregulated autophagy contributes to heart failure, ischemia-reperfusion injury, and atherosclerosis.
D. Infections
Many viruses and bacteria have evolved mechanisms to evade or exploit autophagy. For instance, some pathogens block autophagosome-lysosome fusion to avoid degradation. Conversely, boosting autophagy can enhance pathogen clearance.
E. Autoimmune and Inflammatory Diseases
Autophagy helps maintain immune tolerance and modulates inflammation. Defective autophagy is implicated in diseases like Crohn’s disease, lupus, and rheumatoid arthritis.
Therapeutic Targeting of Autophagy
Given its involvement in various diseases, autophagy is a compelling therapeutic target. Strategies include:
A. Autophagy Inducers
Rapamycin: An mTOR inhibitor that induces autophagy; being explored for neurodegeneration and aging.
Spermidine: A polyamine that promotes autophagy and has shown lifespan-extending effects in model organisms.
Caloric restriction and intermittent fasting: Natural inducers of autophagy with potential health benefits.
B. Autophagy Inhibitors
Chloroquine and hydroxychloroquine: Inhibit autophagosome-lysosome fusion; used in some cancer treatments and autoimmune diseases.
Bafilomycin A1: A more specific inhibitor used in research, but with toxicity that limits clinical use.
The challenge is to selectively modulate autophagy in specific tissues or disease contexts without affecting its essential homeostatic functions.
Autophagy and Aging
As we age, the efficiency of autophagy tends to decline. This decrease can lead to the buildup of damaged proteins and organelles inside cells, which contributes to inflammation and a higher risk of diseases. Poor autophagy function is one of the key factors linked to aging at the cellular level.
Researchers have found that boosting autophagy may help slow down the aging process and improve overall health in later life. In studies with model organisms like mice and worms, increasing autophagy—either through genetic changes or specific drugs—has been shown to extend lifespan and support healthier aging.
One of the most effective natural ways to enhance autophagy is through caloric restriction. This dietary approach activates autophagy and is known to promote longevity. Clinical trials are now exploring drugs that enhance autophagy to see if they can help treat or prevent age-related diseases in humans.
Selective Autophagy
While general autophagy non-selectively engulfs cytoplasmic components, selective autophagy targets specific substrates:
Mitophagy: Removal of damaged mitochondria.
Pexophagy: Degradation of peroxisomes.
Aggrephagy: Clearance of protein aggregates.
Xenophagy: Elimination of intracellular pathogens.
Lipophagy: Breakdown of lipid droplets.
Selective autophagy is mediated by receptor proteins such as p62/SQSTM1, NBR1, and optineurin, which recognize ubiquitinated cargo and bind to LC3 on the phagophore membrane.
Autophagy in Biotechnology and Research
Autophagy plays an important role not just in medicine but also in biotechnology. Scientists study this process in simple organisms like yeast, fruit flies, and mice to better understand how it works. These studies reveal the basic mechanisms of autophagy that are shared across different species, including humans.
To study autophagy in the lab, researchers use specific tools and markers. Autophagy-related genes (ATGs) help show how the process is controlled. Fluorescent markers like GFP-LC3 make it easier to see autophagy inside cells. Scientists also use chemical inducers or inhibitors to understand how different factors affect the process.
New technologies are speeding up autophagy research. Tools like CRISPR allow precise gene editing, while high-throughput screening and single-cell omics help discover new regulators. These advances are helping researchers find potential drug targets and develop new treatments for diseases linked to faulty autophagy.
Conclusion
Autophagy is a fundamental biological process essential for cellular health, organismal survival, and adaptation to stress. Its role extends from intracellular housekeeping to complex functions in immunity, metabolism, and disease prevention. As our understanding deepens, targeting autophagy holds promise for treating a wide array of diseases and potentially extending healthy human lifespan.
However, translating autophagy research into therapies requires a nuanced understanding of its context-specific effects. Enhancing or inhibiting autophagy must be precisely tailored to the disease, stage, and individual. Continued research into the mechanisms and regulation of autophagy will be key to unlocking its full therapeutic potential.
FAQs
- What is autophagy and why is it important ?
Autophagy is the process by which cells break down and recycle their own damaged parts. It helps keep cells clean, healthy, and balanced, especially during stress or lack of nutrients. - How is autophagy related to aging ?
As people age, autophagy becomes less effective, leading to the buildup of waste in cells. This can cause inflammation and increase the risk of age-related diseases. Boosting autophagy may support healthier aging. - Can autophagy help fight diseases ?
Yes, proper autophagy can help prevent or slow diseases such as cancer, neurodegeneration, and infections by removing damaged components and harmful invaders inside cells. - What triggers autophagy in the body ?
Autophagy is triggered by factors like fasting, exercise, low nutrient levels, and certain medications or supplements. - How do scientists study autophagy ?
Researchers use model organisms, genetic tools, fluorescent markers, and advanced technologies like CRISPR and single-cell analysis to study how autophagy works.

